New chemical mechanisms identified on road to cleaner, more efficient combustion
phys.org | March 08, 2018
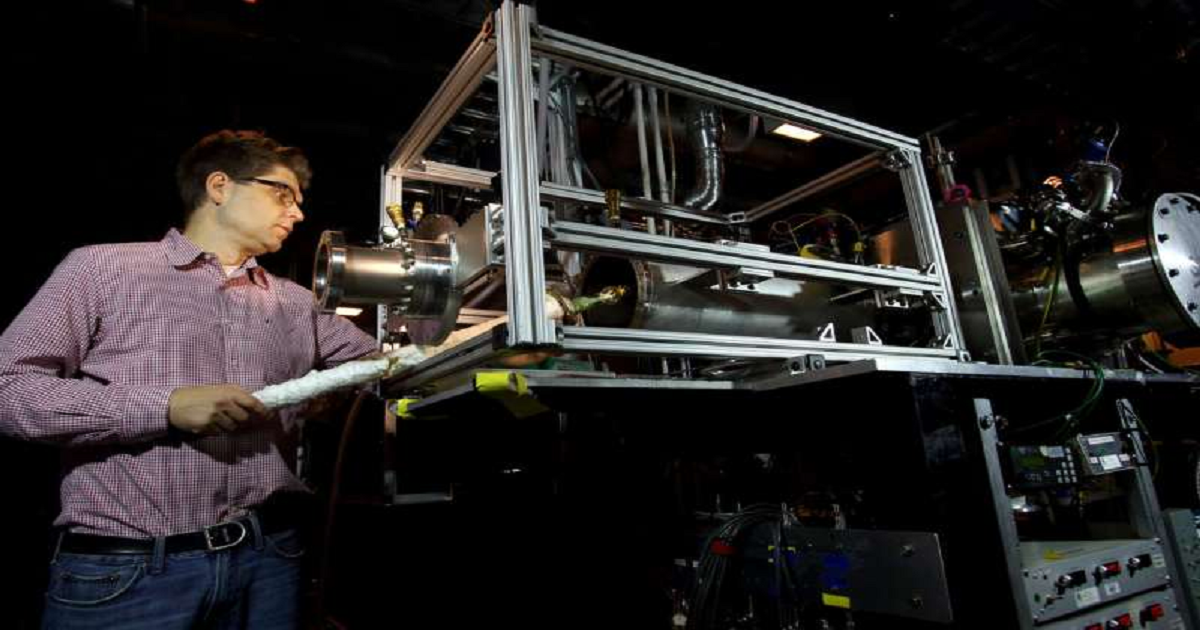
Sandia National Laboratories researchers have identified key chemical mechanisms for the first time that add to the fundamental knowledge of combustion chemistry and might lead to cleaner combustion in engines. Sandia researcher Nils Hansen and former postdoctoral appointee Kai Moshammer focused on low-temperature oxidation of hydrocarbons and other alternative fuels. They identified key chemical intermediates, which are relevant for oxidation reactions at temperatures in the range of 400 to 600 K (260 to 620 degrees Fahrenheit). The chemical nature of the intermediates and their concentrations provides new details on the chemical processes involved in autoignition. Autoignition is a chemical process in which a fuel-air mixture spontaneously ignites. It is commonly explained by theory through a set of self-sustaining and accelerating chain-branching reactions. It is most important for understanding knock in spark-ignition engines. Hansen and Moshammer were among a multi-institution team of researchers whose work was published in a paper titled, "Unraveling the structure and chemical mechanisms of highly oxygenated intermediates in oxidation of organic compounds." The researchers focused on deepening the insights into low-temperature oxidation chemistry of hydrocarbons and other alternative fuels.
"We can run an internal combustion engine today without knowing the details of the chemistry," Hansen said.